{{ price(item.price, true) }}
 Account
Account
 Home
Home
 Categories
Categories
AQA Papers & Mark Schemes
AQA 2023
TEST BANKS
HESI
OCR PAPERS & MARK SCHEMES
AHIP
NCLEX
ATI
EDEXCEL
iHuman
NAPRx
WGU C214
AQA GCSE COMBINED SCIENCE QUESTION PAPERS AND MARK SCHEMES
AQA GCSE QUESTION PAPERS AND MARK SCHEMES
A Level & AS Level Notes
MDC
Exam Elaborations
APEA
TNCC
Wonderlic
NBME
TMC
Prophecy RN
APEX
VATI
NRNP
HESI A2
EMT FISDAP
ITIL
Agile Safe
NSG
NBRC TMC
Straighterline
PAX
Prophecy Medical Surgical
Prophecy Pacu
Rasmussen Pharmacology
BIO 152
NR 667
PN ADULT MED SURG
PN ADULT MEDSURG
NR 507
NR 602
NR
ANCC
CNPR
ENPC
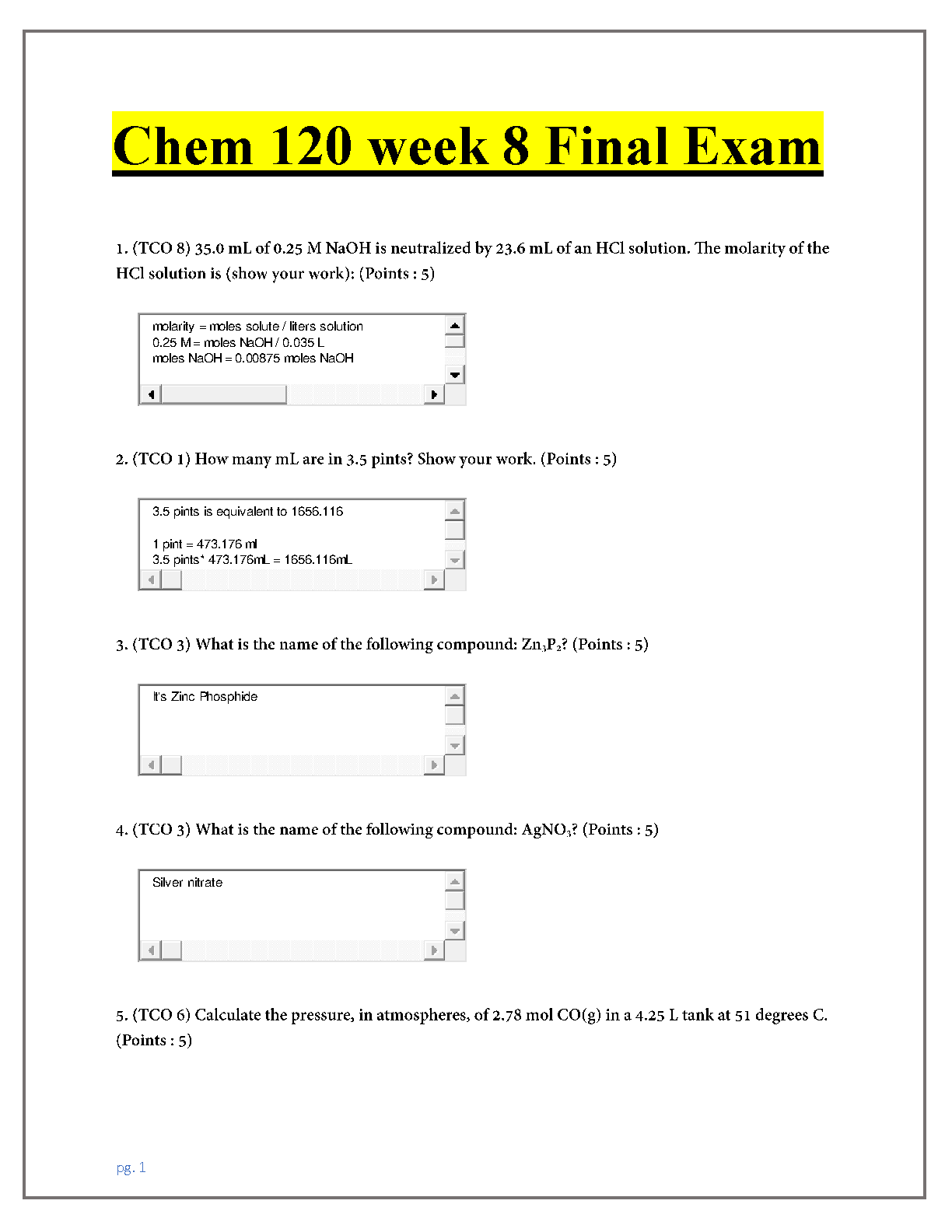
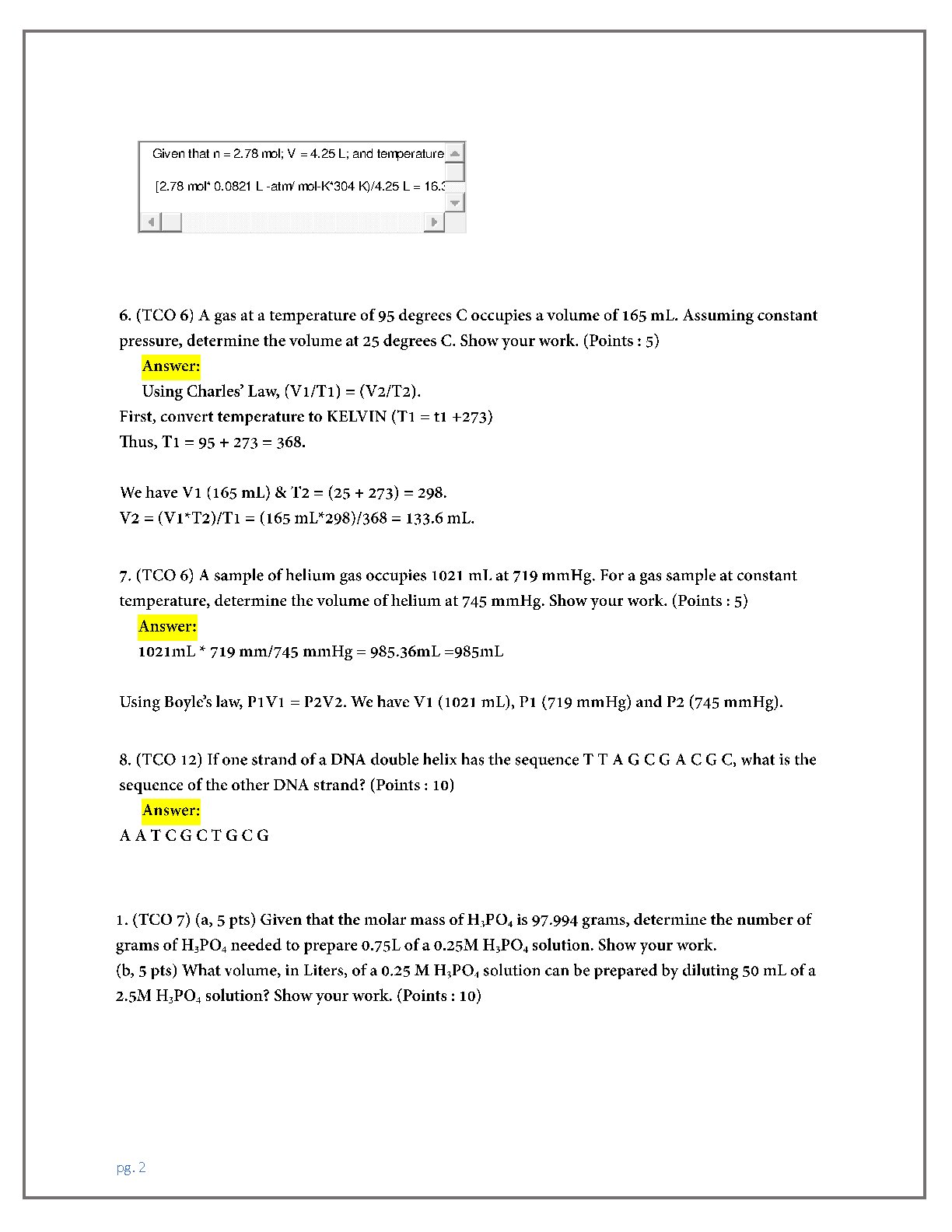
CHEM 103
OCR GCSE
NCCT
CMP
WGU C105
Guam
Crossfit
Texas All Line
Smart Serve
AMLS
ATI Dosage Calculation
MN 551
Case Study
EMT BLOCK
EXCEL CRASH COURSE
USPS
Mental Health PN HESI
NUTRITION 101
RELIAS DYSRHYTHMIA
 Prepaid credits
Prepaid credits
 Blog
Blog
 Collection
Collection
 Privacy policy
Privacy policy
 Terms and conditions
Terms and conditions
 Support
Support
 Languages
Languages
 USD
USD